Cell-Based Product Manufacturing
There are two regulatory paths for cell-based product in Taiwan. One follows the “Medical product (drug)” regulation and the other follows the regulation for the Application of Specific Medical Technique and Equipment (RASMTE). EMO can offer GTP compliant cell-based product services for treatment or clinical trial.
【Medical Product】
- Regulation: Pharmaceutical Act
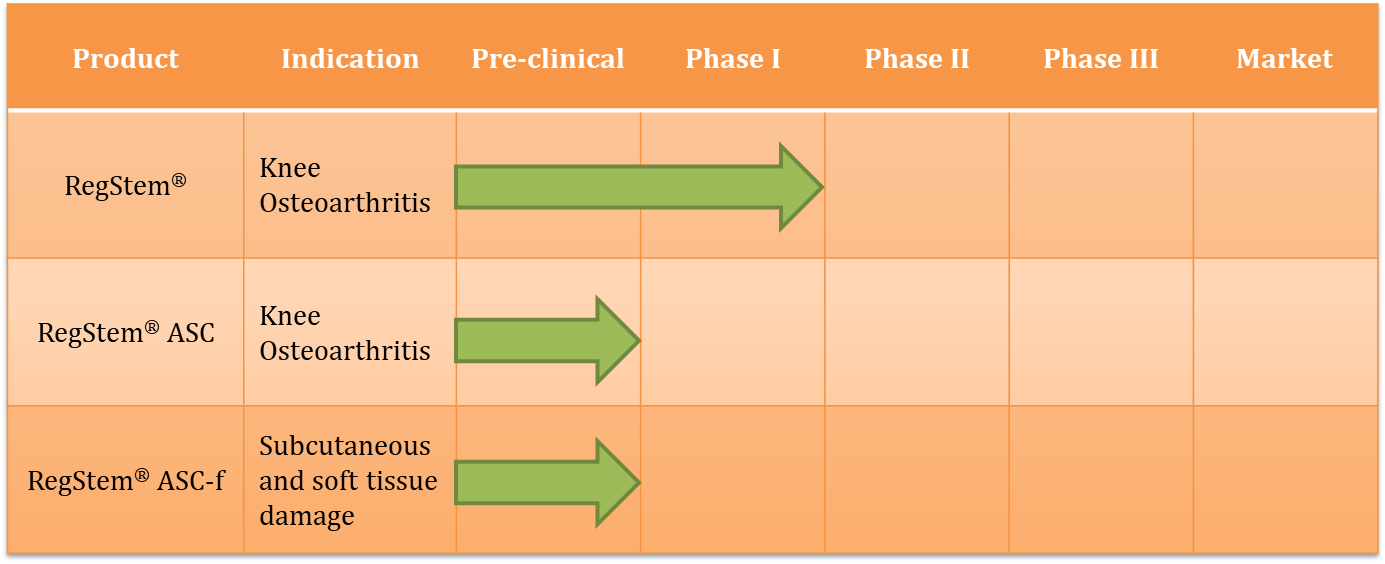
- RegStem®
RegStem® is an autologous adipose stromal cell which derives from infrapatellar fat pad. EMO has established the manufacture processing, release testing and delivery of RegStem®. EMO also developed a proprietary IDO quantification method to control quality and efficacy of cell product. The marketing approval phase 1 trial of RegStem® was completed in 2019. Further related studies are on the going.
RegStem® is an autologous adipose stromal cell which derives from infrapatellar fat pad. EMO has established the manufacture processing, release testing and delivery of RegStem®. EMO also developed a proprietary IDO quantification method to control quality and efficacy of cell product. The marketing approval phase 1 trial of RegStem® was completed in 2019. Further related studies are on the going.
【Medical Technique / Cell Therapy】
- Regulation: The Regulation Governing the Application of Specific Medical Technique and Equipment (RASMTE)
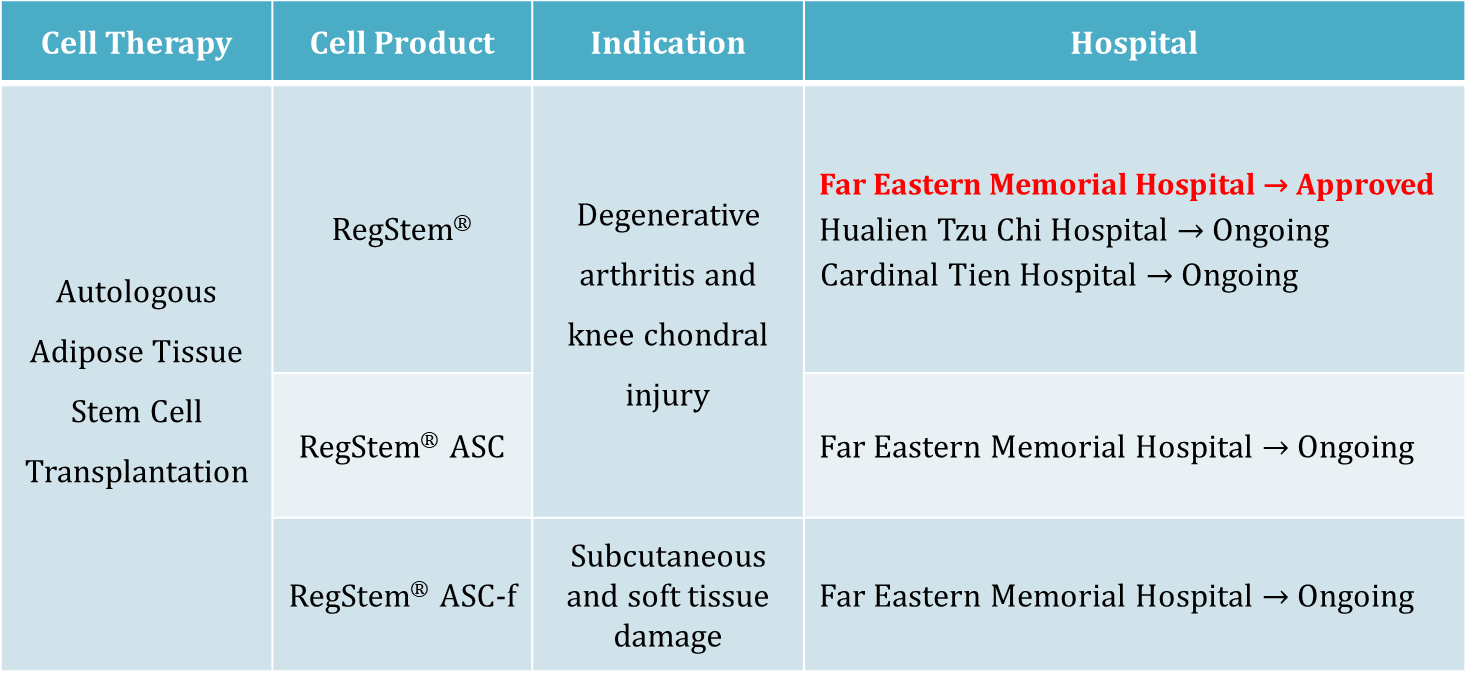
- EMO provides cell therapy of RegStem® series as follows:
- RegStem® is an autologous adipose stromal cell which derives from infrapatellar fat pad for treatment of degenerative arthritis and knee chondral injury.
Based on the manufacturing experience and clinical safety and efficacy from Phase I clinical trial of RegStem®, EMO obtained approval for osteoarthritis treatment from MoHW on December 26, 2019. Far Eastern Memorial Hospital is permitted to treat patient now. Other hospitals are joining soon.
Based on the manufacturing experience and clinical safety and efficacy from Phase I clinical trial of RegStem®, EMO obtained approval for osteoarthritis treatment from MoHW on December 26, 2019. Far Eastern Memorial Hospital is permitted to treat patient now. Other hospitals are joining soon.
- RegStem® ASC is an autologous adipose stromal cell which derives from subcutaneous adipose tissue for treatment of degenerative arthritis and knee chondral injury.
- RegStem® ASC-f is an autologous adipose stromal cell which derives from subcutaneous adipose tissue for treatment of subcutaneous and soft tissue damage.

- TEL:+886-2-2809-6390
- Address:8F., No.29-1, Sec. 2, Zhongzheng E. Rd., Danshui Dist., New Taipei City 251, Taiwan
- E-mail:info@emobio.com
